Brain activation extends the life of mice
In one study, the lifespan of mice was extended when specific brain cells were activated.
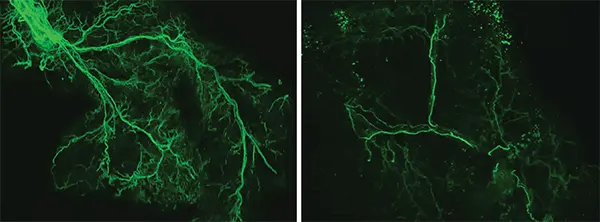
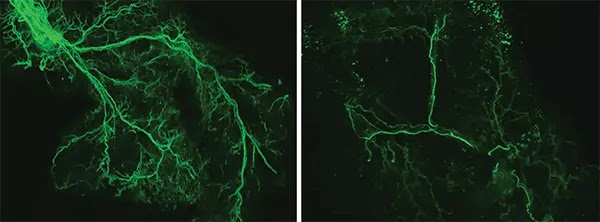
With age, the researchers found that the Ppp1r17 protein tends to leave the nucleus of neurons and, when this happens, the neurons of the hypothalamus send weaker signals. With less use, the wiring of the nervous system throughout the white adipose tissue gradually retracts, and what was once a dense network of interconnected nerves (left) becomes sparse (right). Credit: Kyohei Tokizane
In recent years, research has begun to reveal that the lines of communication between the body’s organs are the main regulators of ageing. When these lines are open, the body’s organs and systems work well together. However, with age, the lines of communication deteriorate and the organs do not receive the molecular and electrical messages they need to function properly.
CONTINUA DEPOIS DA PUBLICIDADELouis identifies, in mice, a critical communication pathway that connects the brain and the body’s fat tissue in a feedback loop that appears to be central to energy production throughout the body. The research suggests that the gradual deterioration of this feedback loop contributes to the increase in health problems typical of natural ageing.
The study – published on January 8 in the journal Cell Metabolism – has implications for the development of future interventions that could maintain the feedback loop for longer and slow down the effects of advancing age.
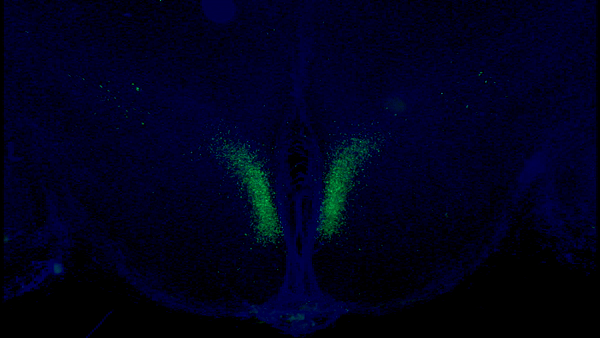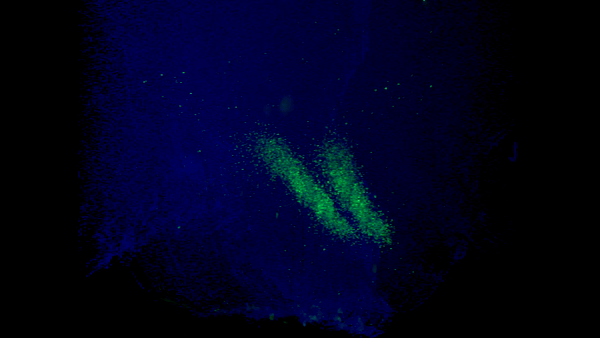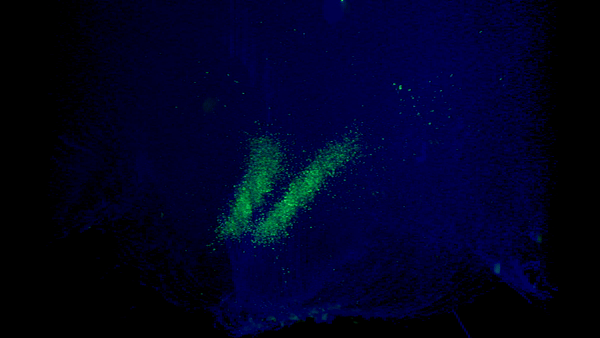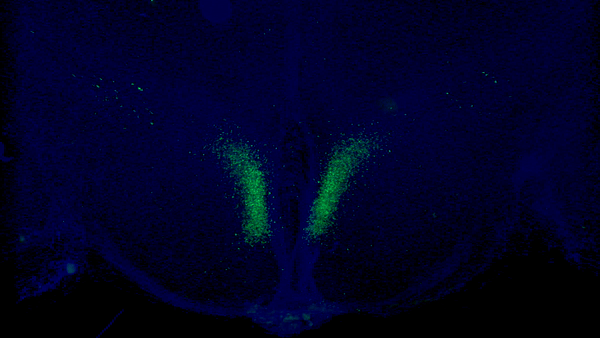
The researchers identified a specific set of neurons in the hypothalamus of the brain which, when active, send signals to the body’s fat tissue to release energy. Using genetic and molecular methods, the researchers studied mice that were programmed to have this communication pathway constantly open after reaching a certain age.
CONTINUA DEPOIS DA PUBLICIDADEThe scientists found that these mice were more physically active, showed signs of delayed ageing and lived longer than mice in which this same communication pathway gradually diminished as part of normal ageing.
“We have demonstrated a way to slow aging and extend healthy lifespan in mice by manipulating an important part of the brain,” said senior author Shin-ichiro Imai, MD, PhD, Theodore and Bertha Bryan Distinguished Professor in Environmental Medicine and professor in the Department of Developmental Biology at the University of Washington. “Demonstrating this effect in a mammal is an important contribution to the field; previous work demonstrating lifespan extension in this way has been carried out in less complex organisms, such as worms and fruit flies.”
These specific neurons, in a part of the brain called the dorsomedial hypothalamus, produce an important protein, Ppp1r17. When this protein is present in the nucleus, the neurons become active and stimulate the sympathetic nervous system, which controls the body’s fight-or-flight response.
CONTINUA DEPOIS DA PUBLICIDADEThe fight-or-flight response is well known to have wide-ranging effects throughout the body, including increased heart rate and slow digestion. As part of this response, the researchers found that neurons in the hypothalamus trigger a chain of events that activates neurons controlling white adipose tissue – a type of fatty tissue – stored under the skin and in the abdominal area.
Activated adipose tissue releases fatty acids into the bloodstream that can be used as fuel for physical activity. Activated adipose tissue also releases another important protein – an enzyme called eNAMPT – which returns to the hypothalamus and allows the brain to produce fuel for its functions.
This feedback loop is fundamental to fueling the body and brain, but it slows down over time. With age, the researchers discovered that the Ppp1r17 protein tends to leave the nucleus of the neurons and, when this happens, the neurons in the hypothalamus send out weaker signals.
CONTINUA DEPOIS DA PUBLICIDADEWith less use, the wiring of the nervous system throughout the white adipose tissue gradually retracts, and what was once a dense network of interconnected nerves becomes sparse. The adipose tissues no longer receive as many signals to release fatty acids and eNAMPT, which leads to fat accumulation, weight gain and less energy to fuel the brain and other tissues.
A new study from Washington University School of Medicine in St. Louis has identified a fundamental feedback loop between the brain and adipose tissue that governs aging in mice. Key neurons (shown in green) in the brain’s dorsomedial hypothalamus activate adipose tissue to produce cellular fuel. When these specific neurons are activated in older mice, they live longer than control mice. Credit: Kyohei Tokizane
The researchers, including first author Kyohei Tokizane, PhD, a staff scientist and former postdoctoral researcher in Imai’s lab, found that when they used genetic methods in aged mice to maintain Ppp1r17 in the nucleus of neurons in the hypothalamus, the mice were more physically active – with a greater number of wheel races – and lived longer than the control mice.
CONTINUA DEPOIS DA PUBLICIDADEThey also used a technique to directly activate these specific neurons in the hypothalamus of aged mice and observed similar anti-aging effects.
On average, the upper limit of a typical laboratory mouse’s lifespan is around 900 to 1,000 days, or about 2.5 years. In this study, all the control mice that aged normally died at 1,000 days of age.
Those that underwent interventions to maintain the brain adipose tissue feedback loop lived 60 to 70 days longer than the control mice. This translates into an increase of around 7% in life expectancy. In people, a 7% increase on a life expectancy of 75 years translates into around five years more.
CONTINUA DEPOIS DA PUBLICIDADEThe mice that received the interventions were also more active and looked younger – with thicker, shinier fur – at older ages, which suggests longer in better health too.
Imai and his team continue to investigate ways of maintaining the feedback loop between the hypothalamus and adipose tissue. One way they are studying involves supplementing mice with eNAMPT, the enzyme produced by adipose tissue that returns to the brain and feeds the hypothalamus, among other tissues.
When released from adipose tissue into the bloodstream, the enzyme is packed into compartments called extracellular vesicles, which can be collected and isolated from the blood.
CONTINUA DEPOIS DA PUBLICIDADE“We can envision a possible anti-aging therapy that involves administering eNAMPT in various ways,” said Imai. “We have already shown that administering eNAMPT in extracellular vesicles increases cellular energy levels in the hypothalamus and extends the lifespan of mice. We look forward to continuing our work investigating ways to maintain this central feedback loop between the brain and the body’s adipose tissues in ways that we hope will increase health and life expectancy.”
This article has been republished from Washington University inSt.Louis. Read the original article.
", type: "opt-in", position: "bottom-left", theme: "classic", palette: { popup: { background: "#1e293b", text: "#fff" }, button: { background: "#ad56f0", text: "#fff" } }, content: { message: "Este site usa cookies para melhorar sua experiência. Ao continuar navegando neste site, você concorda com o uso de cookies. Consulte nossa ", link: "Política de privacidade", allow: "Aceitar", deny: "Negar", href: "https://www.insoniaoculta.com.br/p/politica-de-privacidade.html" }, onInitialise: function(status) { if(status == cookieconsent.status.allow) myScripts(); }, onStatusChange: function(status) { if (this.hasConsented()) myScripts(); } }) });
function myScripts() {
// Paste here your scripts that use cookies requiring consent. See examples below
CONTINUA DEPOIS DA PUBLICIDADE// Google Analytics, you need to change 'UA-00000000-1' to your ID (function(i,s,o,g,r,a,m){i['GoogleAnalyticsObject']=r;i[r]=i[r]||function(){ (i[r].q=i[r].q||[]).push(arguments)},i[r].l=1*new Date();a=s.createElement(o), m=s.getElementsByTagName(o)[0];a.async=1;a.src=g;m.parentNode.insertBefore(a,m) })(window,document,'script','//www.google-analytics.com/analytics.js','ga'); ga('create', 'UA-00000000-1', 'auto'); ga('send', 'pageview');
// Facebook Pixel Code, you need to change '000000000000000' to your PixelID !function(f,b,e,v,n,t,s) {if(f.fbq)return;n=f.fbq=function(){n.callMethod? n.callMethod.apply(n,arguments):n.queue.push(arguments)}; if(!f._fbq)f._fbq=n;n.push=n;n.loaded=!0;n.version='2.0'; n.queue=[];t=b.createElement(e);t.async=!0; t.src=v;s=b.getElementsByTagName(e)[0]; s.parentNode.insertBefore(t,s)}(window, document,'script', 'https://connect.facebook.net/en_US/fbevents.js'); fbq('init', '000000000000000'); fbq('track', 'PageView');
}
CONTINUA DEPOIS DA PUBLICIDADEQuer continuar acompanhando conteúdos como este? Junte-se a nós no Facebook e participe da nossa comunidade!
Seguir no Facebook